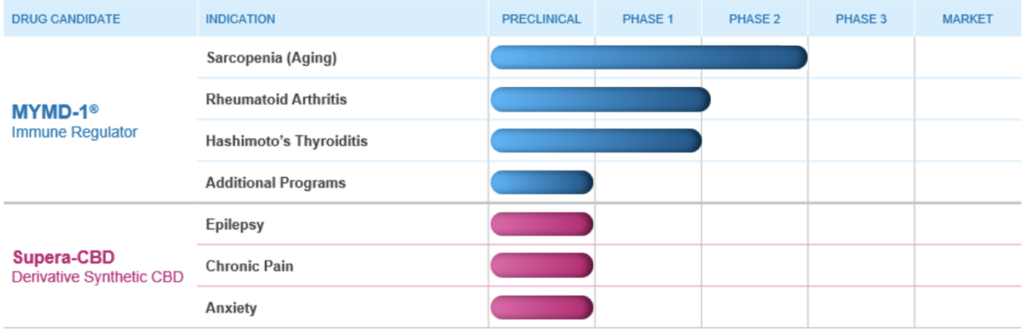
Recently, MyMD announced positive, statistically significant Phase 2 study results in participants with sarcopenia/frailty which showed MYMD-1® reduced TNF-α, IL-6 and sTNFR1, biomarkers which are common to a number of chronic inflammatory diseases, and met all safety and tolerability endpoints. The company is now planning to initiate Phase 3 trials. If approved, MYMD-1® has the potential to be the first drug approved by FDA for the sarcopenia, an age-related decline in muscle mass and physical function which leads to greater risk of hospitalization, disability, and death. MyMD has now submitted and received approval for 3 Investigational New Drug Applications for MYMD-1®, and is advancing these clinical programs in chronic inflammatory conditions including Sarcopenia, RA and Hashimoto’s Thyroiditis
We will also execute studies of Supera-CBD that will enable submission of an Investigational New Drug Application (IND) to the FDA for a Phase 1 clinical trial in epilepsy, chronic pain, and anxiety disorders.